
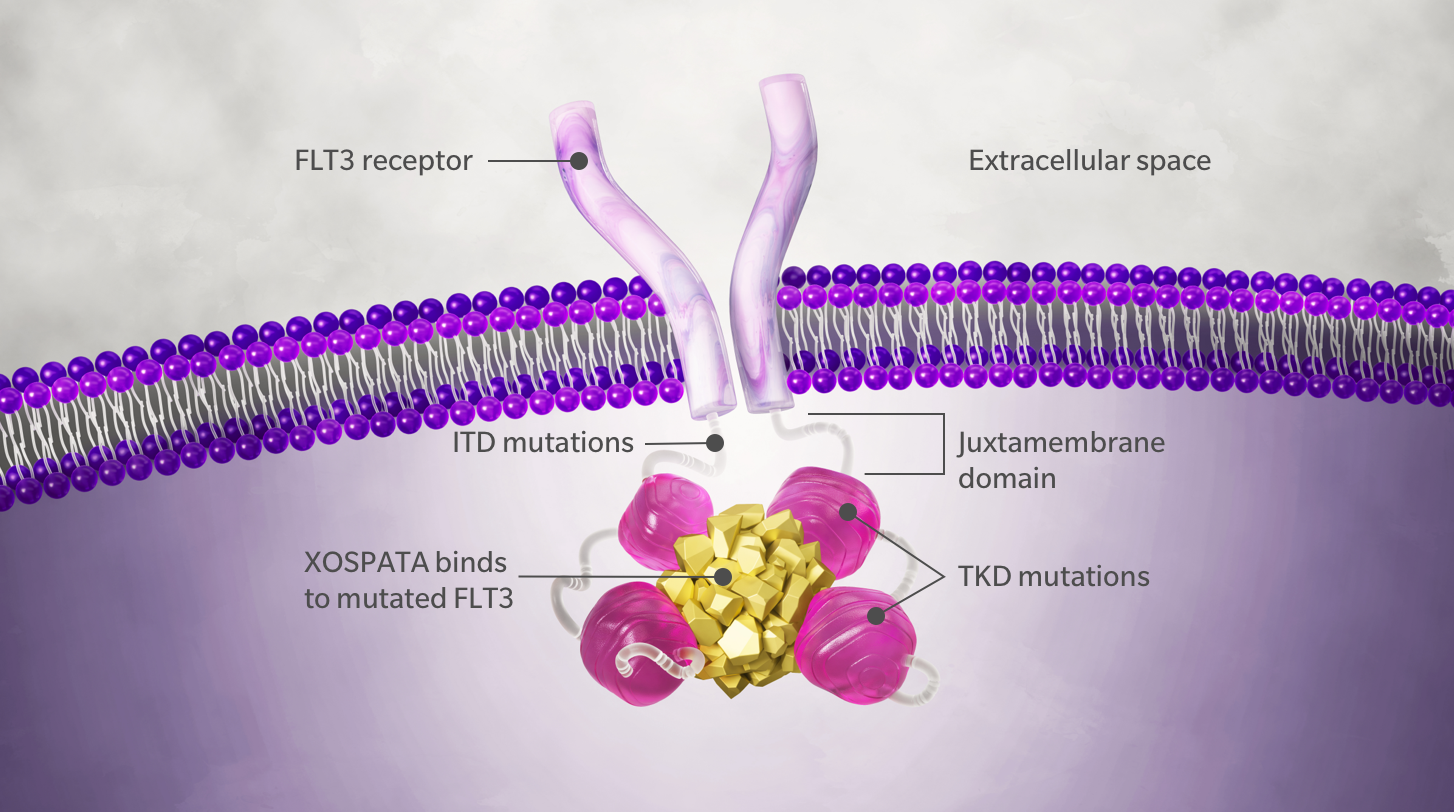
Findings included hepatic, bone marrow/hematologic (primarily platelet), lymphoid organ, and neuronal toxicities, and increased numbers of cells of epithelial and phagocytic origin in metaphase arrest. T-DM1 demonstrated a double-punch mechanism, by which trastuzumab allowed selective delivery of DM1 to HER2-overexpressing cells while retaining its ability to induce ADCC and inhibition of HER2. In addition, T-DM1 and DM1 safety profiles were similar and consistent with the mechanism of action of DM1 (i.e., microtubule disruption). Mechanisms of action of T-DM1 Binding of T-DM1 to HER2 triggers entry of the HER2-T-DM1 complex into the cell via receptor-mediated endocytosis 12,13. Mechanism of Action Upon binding to sub-domain IV of the HER2 receptor, ado-trastuzumab emtansine undergoes receptor-mediated internalization and subsequent lysosomal degradation, resulting in the intracellular release of DM1. This suggests that at least two-fold higher doses of the cytotoxic agent are tolerated in T-DM1, supporting the premise of ADCs to improve the therapeutic index. Source: Highlights of Prescribing Information (USA) ado-trastuzumab emtansine (T-DM1 Kadcyla/Genentech/Roche). In contrast, DM1 was only tolerated up to 0.2 mg/kg (1600 μg DM1/m 2). Currently T-DM1 is being tested in multiple clinical trials. Mechanisms of action of T-DM1 T-DM1 has multiple mechanisms of action, from the selective delivery of DM1 to HER2-positive tumour cells through to trastuzumab-mediated inhibition of HER2. Trastuzumab-DM1 (T-DM1, trastuzumab emtansine) is designed to combine the clinical benefits of trastuzumab with a potent microtubule-disrupting drug, DM1 (a maytansine derivative). T-DM1 is another HER2 directed antibody therapy recently approved for treatment of HER2-positive breast cancer. Trastuzumab (Herceptin) is currently used as a treatment for patients whose breast tumors overexpress HER2/ErbB2. T-DM1 was well tolerated at doses up to 40 mg/kg (~ 4400 μg DM1/m 2) and 30 mg/kg (~ 6000 μg DM1/m 2) in rats and monkeys, respectively. Mechanism of action and preclinical data. The approval of ado-trastuzumab emtansine (T-DM1) for clinical use represented a turning point both in HER2-positive breast cancer treatment and antibody. Therefore, antigen-dependent and non-antigen-dependent toxicity was evaluated in monkeys and rats, respectively, in both single- and repeat-dose studies toxicity of DM1 was assessed in rats only. T-DM1 binds primate ErbB2 and human HER2 but not the rodent homolog c-neu. Here, we present results from preclinical studies characterizing the toxicity profile of T-DM1, including limited assessment of unconjugated DM1. The therapeutic premise of ADCs is based on the hypothesis that targeted delivery of potent cytotoxic drugs to tumors will provide better tolerability and efficacy compared with non-targeted delivery, where poor tolerability can limit efficacious doses. Trastuzumab emtansine (T-DM1) is the first antibody-drug conjugate (ADC) approved for patients with human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer.


 0 kommentar(er)
0 kommentar(er)
